Sarepta Crashes After Second Death On Gene Therapy Drug
Sarepta Therapeutics shares suffered their steepest drop in years on Monday morning after a second teenage patient died following treatment with Elevidys, the biotech's one-time gene therapy for Duchenne muscular dystrophy (DMD).
"These steps follow a second reported case of acute liver failure (ALF) resulting in death. The cases of ALF to date have both occurred in non-ambulatory individuals with Duchenne. Sarepta extends its deepest sympathies to the affected families and care teams," Sarepta wrote in a press release.
In response, Sarepta suspended 2025 revenue guidance and will "take a careful look" at its costs moving forward, CEO Doug Ingram told Wall Street analysts on a call. The second loss of life will intensify the Food and Drug Administration's scrutiny of the FDA‑approved, prescription gene therapy available commercially in the U.S. and several other countries.
Goldman healthcare analyst PJ Gallo told clients earlier:
Second Patient Death on ELEVIDYS Drives Weakness: The company reported a second case of acute liver failure that resulted in death in a non-ambulatory individual with DMD who received ELEVIDYS. Recall the first death was reported back in mid-March. Shipments of ELEVIDYS for non-ambulatory patients were suspended as a result. And dosing for the company's ENVISON study was voluntarily paused.
Cantor Fitzgerald analyst Kristen Kluska stated:
"We think a second death is going to dramatically impact physician, caregiver and patient decisions moving forward."
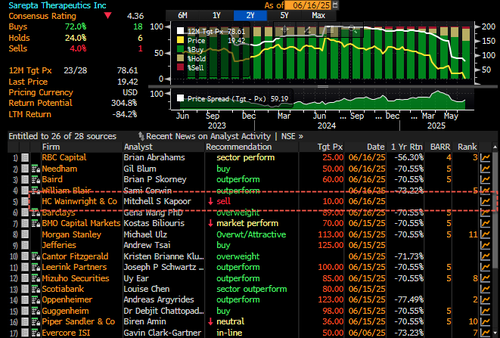
There were notable Street actions: SRPT Sarepta Cut to Neutral at Piper Sandler; PT $36, Sell at HC Wainwright; PT $10, Neutral at Piper Sandler; PT $36, Market Perform at BMO; PT $70
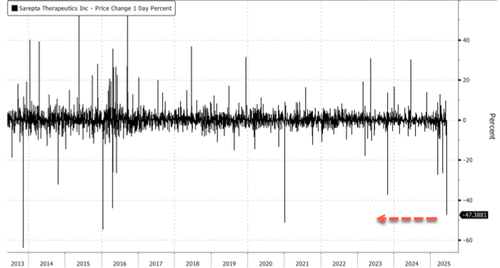
Sarepta shares crashed as much as 48% in the U.S. morning, the most since Jan. 8, 2021.
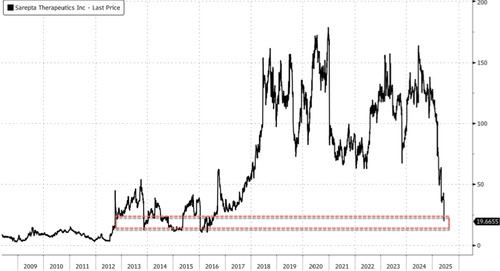
Roundtrip.
Thanks for playing.
Loading...