Aging Spreads Through The Body Like An Infection, And This Protein Could Be To Blame

Abstract representation of aging (senescent) cells, depicting the gradual decline and transformation of biological structures over time, symbolizing the natural process of cellular deterioration and renewal. (© dang - stock.adobe.com)
Scientists Discover How A ‘Redox Switch’ Turns Youthful Cells Old (And How to Stop It) In A NutshellSEOUL, South Korea — Researchers have identified a molecular culprit behind one of biology’s most puzzling phenomena: how cellular aging spreads from one part of the body to another, potentially accelerating the overall aging process.
A new study published in the journal Metabolism reveals that a protein called HMGB1 acts as a redox-sensitive “aging messenger,” carrying signals that can turn healthy cells into aged, dysfunctional ones — but only when it’s in the right chemical state.
How HMGB1 Protein Controls Cellular AgingHMGB1 normally resides inside the nucleus of cells, where it helps organize DNA. But when cells become stressed or aged, a state scientists call “senescent,” they release this protein into their surroundings. Once outside the cell, HMGB1 can exist in different chemical forms depending on how much oxygen it has been exposed to.
The research team from Korea University College of Medicine discovered that only the “reduced” form of HMGB1 acts as an aging accelerator. Much like how rust depends on environmental conditions, the chemical surroundings determine whether this protein triggers damage. The reduced form, which hasn’t been exposed to much oxygen, can bind to cellular receptors and activate aging pathways. The oxidized form, by contrast, loses this ability.
To test this, the researchers conducted extensive lab experiments using human lung, kidney, skin, and muscle cells. When treated with the reduced form of HMGB1 for several days, healthy cells began showing classic signs of aging: they stopped dividing, expressed senescence markers like p21 and p16, and began releasing inflammatory molecules.
Cells treated with the oxidized form remained healthy and continued to divide normally. The effect was consistent across all tested cell types.
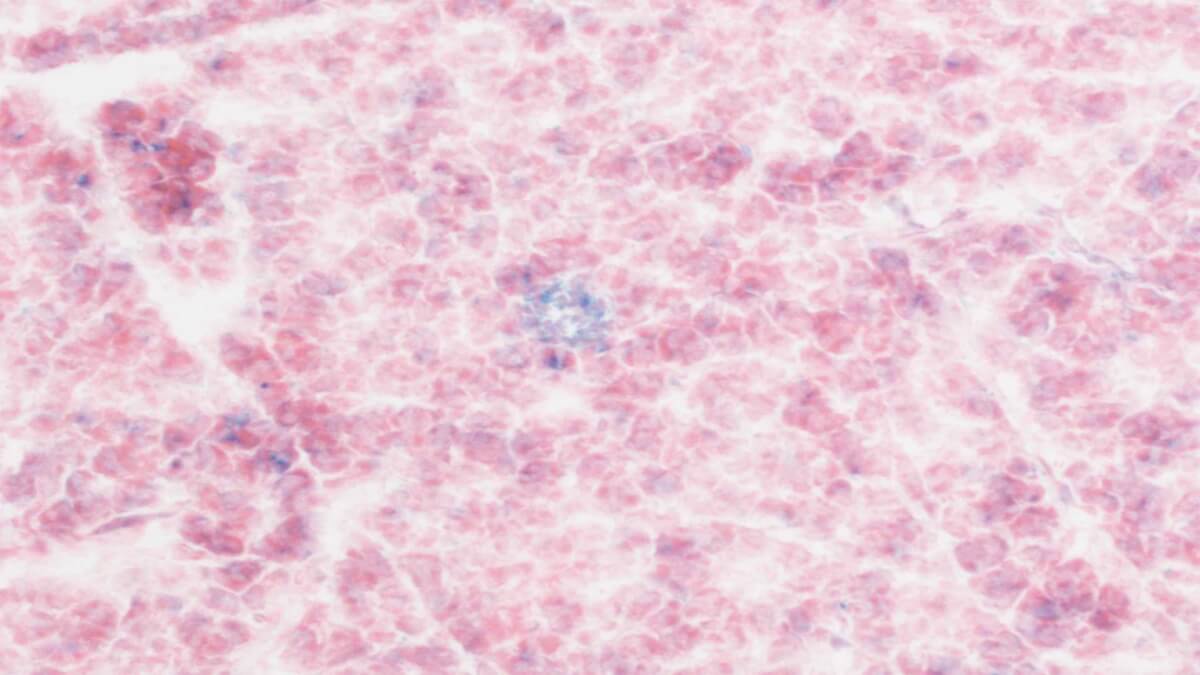 Senescent cells (blue) accumulate as we age. CAR T cells can be programmed to seek them out and destroy them. The image above shows healthy pancreatic tissue samples from an old mouse treated with CAR T cells as a young pup. (Credit: Cold Spring Harbor Laboratory)
Why Chemical State Determines Aging Effects
Senescent cells (blue) accumulate as we age. CAR T cells can be programmed to seek them out and destroy them. The image above shows healthy pancreatic tissue samples from an old mouse treated with CAR T cells as a young pup. (Credit: Cold Spring Harbor Laboratory)
Why Chemical State Determines Aging Effects
Advanced genetic sequencing revealed what was happening inside the cells. The reduced form of HMGB1 activated molecular signaling cascades, specifically the JAK/STAT and NF-κB pathways, that are known to promote inflammation and cellular aging.
When researchers blocked these pathways with existing drugs, the aging effects were eliminated, confirming that the reduced form acts through these mechanisms. According to the study, “Extracellular ReHMGB1, but not its oxidized form, robustly induced senescence-like phenotypes across multiple cell types and tissues.”
This creates a potentially harmful cycle: as people age and accumulate more senescent cells, those cells release more reduced HMGB1, which then causes even more healthy cells to become senescent. In this way, aging may propagate through the body via molecular messengers.
Mouse Studies Reveal Potential Anti-Aging TherapiesThe team found that these cellular effects translated into real-world consequences in animals. When young, healthy mice were injected with the reduced form of HMGB1 at a dose of 5 mg per kilogram of body weight, the animals developed aging-like symptoms within one week. Muscle tissue showed elevated levels of senescence markers, and blood tests revealed a spike in inflammatory cytokines linked to aging.
In a separate experiment, 15-month-old mice with muscle injuries were treated with antibodies that block HMGB1. Compared to untreated mice, those receiving the antibody showed better muscle healing, reduced inflammation, and improved physical performance. They gripped harder, ran farther on treadmills, and healed faster, indicating that interfering with HMGB1 signaling helped reduce age-related dysfunction.
Blood samples from older adults (ages 70–80) also contained significantly higher levels of the reduced form of HMGB1 compared to people in their 40s. Similar age-related increases were seen in laboratory mice.
 (© vesvocrea – stock.adobe.com)
Future Applications for Human Anti-Aging Medicine
(© vesvocrea – stock.adobe.com)
Future Applications for Human Anti-Aging Medicine
The findings open several potential avenues for anti-aging therapies. Drugs could be developed to block the reduced form of HMGB1 from binding to its receptor (called RAGE), to promote its oxidation into an inactive form, or to block the inflammatory pathways it activates.
Some of these strategies may already be feasible. The study showed that existing drugs targeting the JAK pathway, already approved to treat certain autoimmune diseases, can prevent HMGB1-induced aging in cells.
This research shifts how scientists understand aging. Rather than being an isolated process occurring independently in each cell, aging may spread through tissues and organs via chemical messengers. The study describes the reduced form of HMGB1 as a “pro-geronic factor” — a molecule that promotes aging — and highlights it as a promising target for future therapies.
For anyone hoping to age more gracefully, this protein may hold the key to both the problem and future therapeutic strategies.
Disclaimer: The findings described in this article are based on laboratory and animal studies. While promising, they have not yet been tested in clinical trials for anti-aging purposes in humans. Always consult qualified medical professionals before considering experimental therapies.
Paper Summary MethodologyResearchers used several experimental systems to explore the effects of different HMGB1 forms on aging. They exposed human cells to reduced or oxidized HMGB1 and analyzed aging-related changes using genetic and protein markers. In mouse studies, they injected young mice with reduced HMGB1 and examined signs of aging. They also tested antibody treatments in older mice with muscle injuries and measured healing and performance. Blood samples from humans of different ages were analyzed for HMGB1 levels.
ResultsThe reduced form of HMGB1 caused healthy human cells to stop dividing and produce inflammatory molecules associated with senescence. Oxidized HMGB1 had no such effect. In mice, injections of reduced HMGB1 led to aging-like changes in muscle and liver tissue within one week. Blocking HMGB1 in injured older mice reduced inflammation, improved muscle regeneration, and enhanced strength and endurance. Older humans had significantly more reduced HMGB1 in their blood than younger individuals.
LimitationsThe experiments were conducted in cell cultures and mice, which may not fully reflect human biology. The doses and timeframes used may not represent natural conditions. Human blood sample data came from a relatively narrow age range. Anti-HMGB1 treatment was only tested in muscle injury, not across broader aging-related contexts.
Funding and DisclosuresThe research was funded by the National Research Foundation of Korea and the USDA-ARS. The authors reported no financial conflicts of interest.
Publication DetailsThe study, titled “Propagation of senescent phenotypes by extracellular HMGB1 is dependent on its redox state,” was published in Metabolism, Volume 168 (2025). The lead author is Ok Hee Jeon of Korea University College of Medicine, with collaborators in South Korea and the United States.